Introduction
Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused many complications, the invention of coronavirus disease 2019 (COVID-19) vaccines has also brought about several adverse events from common side effects to unexpected and rare ones (1). Common vaccine-related side adverse reactions are those manifested locally or systematically following any vaccine, including COVID-19 vaccines. Local reactions include erythema, tenderness, induration, and, rarely, abscess formation at the injection site. In contrast, systemic side effects include fever, chills, headache, cough, coryza, and rarely anaphylactic reactions (2). However, specific side effects, known as adverse events of special interest (AESI), could manifest as autoimmune diseases or involve various organs, such as renal, dermatologic, hematologic, lymphatics, ocular, gastrointestinal, cardiovascular, and neurologic systems, are unusual and need more evaluation (1). Some adverse events have never happened with other previously administered vaccines (Figure 1). Examples are those related to the hyperthrombotic condition induced by the COVID-19 vaccines that have been discussed in several articles (3). Some rare adverse events have been discussed among the reported side effects of COVID-19 vaccines. Table 1 encompasses the reported frequencies of these adverse events. First, their proposed pathophysiology is described, and the possible diagnostic approaches along with recommended treatment options are demonstrated. Finally, most of the following adverse events discussed in this paper are very uncommon. Since there is no formal evidence that vaccines are responsible in most cases, it has to be considered that establishing a cause-effect relationship is challenging and needs more investigation into the actual occurrence of the complication and the underlying pathophysiology.
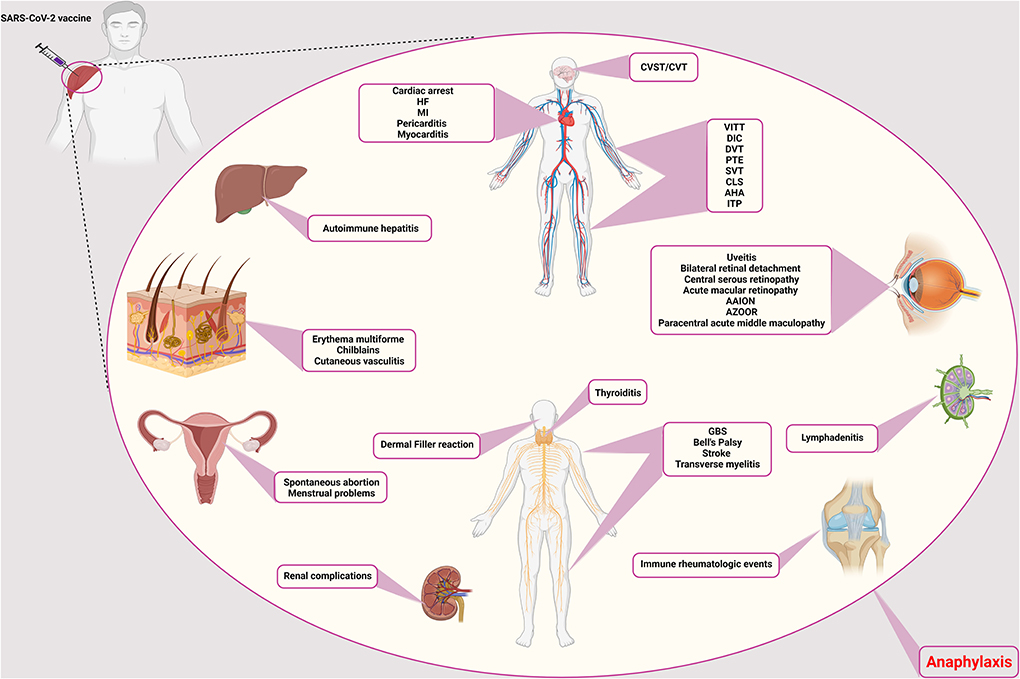
Figure 1. Rare adverse events of COVID-19 vaccination. The side effects of the COVID-19 vaccines are different and can affect different systems and tissues in the body. Vascular side effects have been seen in the brain, vascular system of the limbs, abdomen, and heart, including CVST/CVT, VITT, DIC, DVT, PTE, CLS, AHA, ITP, SVT, cardiac arrest, HF, MI, pericarditis, and myocarditis, respectively. Ocular involvement includes uveitis, bilateral retinal detachment, central serous retinopathy, acute macular retinopathy, AAION and AZOOR, and paracentral acute middle maculopathy. The thyroid gland can also cause thyroiditis. Neurological side effects such as GBS, Bell's palsy, stroke, and transverse myelitis have also been observed. It causes filler on the face. In addition to facial involvement, skin infections such as erythema multiforme, chilblains, and cutaneous vasculitis have also been reported. It causes autoimmune hepatitis in the liver and has caused many complications for the kidneys. Symptoms of immune rheumatologic events have also been observed in some patients. Lymphadenitis is one of the immune complications in the lymph nodes. In addition to the above, it also causes spontaneous abortion and menstrual problems in women. CVST/CVT, Cerebral venous sinus thrombosis/Cerebral venous thrombosis; VITT, Vaccine-induced immune thrombotic thrombocytopenia; DIC, Disseminated intravascular coagulation; DVT, Deep vein thrombosis; PTE, Pulmonary thromboendarterectomy; CLS, Capillary leak syndrome; AHA, Acquired hemophilia A; ITP, Immune thrombocytopenic purpura; SVT, Supraventricular tachycardia; HF, Heart failure; MI, Myocardial infarction; AAION, Arteritic anterior ischemic optic neuropathy; AZOOR, Acute zonal occult outer retinopathy; GBS, Guillain-Barré syndrome.
Autoimmune complications
Acquired hemophilia A
Acquired hemophilia A (AHA) is a rare autoimmune-hematologic disorder within the immune system that is aroused by a trigger to produce auto-antibodies against clotting factor VIII (FVIII inhibitor) (4). It is suggested to be associated with predisposing factors, such as autoimmune diseases, drugs, pregnancy, infections, and malignancy. AHA major presentations include bruise or ecchymosis in the skin, mainly in the extremities, but it may progress to muscles and mucosal layers. However, joint involvement is not included despite the hereditary type (5). Recently, few case reports have introduced SARS-CoV-2 mRNA vaccines as a possible trigger for AHA without other predicting conditions (6–8). There are also several reports on exhibiting AHA following SARS-CoV-2 infection (5). Presumed pathophysiology can be the antigenic mimicry of SARS-CoV-2 and FVIII, leading to uncontrollable degradation of FVIII. Autoimmune response may be conducted by various underlying causes such as particular genetic polymorphism and activation of previously existing autoimmune B/T cells (9). Laboratory confirming evidence includes prolonged activated partial thromboplastin time (aPTT), decreased level of FVIII, and elevated FVIII inhibitor (4). Mentioned cases diagnosed with vaccination caused AHA within days to weeks following receiving first/second doses of Moderna (mRNA1273) or Pfizer-BioNTech (BNT162b2) vaccines (5, 6, 8, 9). Cases were mainly discharged after a few days of treatment. AHA treatment methods consist of two major issues: (1) homeostasis normalization. First, it is vital to complete the coagulation cascade with recombinant activated factor VII (rFVIIa) and activated prothrombin complex concentrate (aPCC); and (2) immune suppressive therapy should be conducted by corticosteroids (mainly high dose prednisone), cyclophosphamide, and rituximab (in refractory cases) (4). Given the very low incidence of AHA following the COVID-19 vaccines, which might be a coincidental event (8), it can be challenging to define a cause-effect relation between AHA and vaccination, and it is not wise to limit the COVID-19 vaccination. Instead, it is better to continue investigations to clarify whether there is any cause-effect relation.
Immunomediated thrombocytopenia
Immunomediated thrombocytopenia (ITP) is an autoimmune disorder in which platelets destroy by autoantibodies. Etiology primarily includes autoimmunity but it can also occur secondary to viral infections and even vaccination. In the past years, after the beginning of the COVID-19 vaccination, several ITP cases have been reported, mainly following mRNA vaccines (10). The adjusted RR for ITP, 0–27 days following ChAdOx1 vaccination, was 5.77 (estimated incidence: 1.13 events per 100,000 doses). Nevertheless, there was no association between BNT162b2 vaccine and ITP (11). In terms of de novo ITP, which happens within days, hypothesized pathophysiology encompasses molecular mimicry and cross-reaction of antibodies against vaccine components and platelet antigens (9). Albeit, in case of a flare-up of pre-existing ITP occurring in hours, the underlying mechanism amplifies prior immune response (9). It is worth noting that the measured incidence of secondary ITP after vaccination has been lower than expected ITP in the general population so far (12). However, as the exact differentiation of coincidental ITP and ITP related to vaccination is impossible, immediate treatment should be performed to prevent severe outcomes (12). Patients present with mild-to-severe symptoms due to the platelet count. In moderate thrombocytopenia, symptoms generally include petechiae, purpura, bruising, and mucosal bleeding (13). Platelet count under 5,000/μL is life-threatening with a high risk of intracranial hemorrhage (ICH) (13). Treatment typically consists of IV fluids, corticosteroids, IVIG, platelet transfusion, rituximab, and thrombopoietic agents (e.g., romiplostim, eltrombopag) (13). A better approach has been suggested to start the initial treatment with corticosteroids (e.g., dexamethasone, methylprednisone) and IVIG (13). Furthermore, if the platelet count did not rise, other agents could be added. Since rituximab responds in about 8 weeks and alters the body's reaction to the vaccine, it should be excluded from initial treatment (13). Although many studies have reported ITP as a possible complication of COVID-19 infection (14–21), fewer have reported ITP occurring following SARS-CoV-2 vaccines, and its risk is too less than the infection-induced ITP (22).
Anaphylaxis
There are two types of adverse events following immunization (AEFI) after receiving vaccines; non-allergic reactions are normal expected immune responses, including pain in the injection site, nausea, fever, chill, and fatigue, while allergic reactions are caused by body hypersensitivity to vaccines' adjuvant (not an active ingredient) such as excipients. Allergic reactions may involve any body part and can exhibit mild-severe symptoms in different patients, including flush, pruritus, facial edema, tachycardia, laryngeal edema, and diarrhea (23). Anaphylaxis is perceived as an allergic AEFI with a very low incidence among vaccine receivers (1 in a million doses) and a higher rate in women, which is increasingly garnering attraction these days (24). Underlying pathophysiology has been proposed to be IgE-mediated type one hypersensitivity (25). However, there are a few other explanations as well. For example, complement activation pseudo-allergy (CARPA) is mainly instigated through C3a and C4a components without the involvement of IgE. Direct interaction of double-stranded RNA applied in vaccines and mast cells is also possible, but it is reported to be unlikely due to a lack of evidence in previous in vitro studies (26). For an IgE-mediated allergy, there should be prior contact with the allergen. To further decipher, polyethylene glycol (PEG) or Macrogol and polysorbate 80 have been reported as the most suspected component for vaccine-related anaphylaxis. PEG2000 has been utilized in SARS-CoV-2 mRNA vaccines as an excipient to facilitate mRNA delivery into cells. Note that it has a widespread application in medications and foods. Moreover, polysorbate 80 is a PEG-derived molecule with a lower weight recruited in many vaccines and medications (23). Their similar structures might lead to a cross-reactivity. It is worth noting that most of the reported cases had a prior allergy to foods, drugs, or vaccines (27).
Anaphylaxis mainly occurs in the first 15 min after vaccination. Symptoms mainly encompass sensation of throat closure, upper airway swelling, nausea-vomiting, tachycardia, difficulty breathing without wheezing or stridor, angioedema, hypotension, and dry cough (27). Diagnosis is primarily clinical and needs immediate action because it can be potentially fatal despite its very low incidence. Treatment includes urgent intra-muscular injection of epinephrine (0.01 mg/kg) and assessing airway, breathing, circulation, and mental status at the same time (23). Epinephrine injection repeats every 5–15 min if the symptoms stay resilient up to the maximum allowed dose of 0.5 mg in adults (0.3 mg in prepubertal children) (23). Antihistamine and glucocorticoids are second-line drugs mainly for skin and mucosal reactions. Note that PEG or polysorbate has been added to some of this medication which should not use as a treatment in patients with suspected allergy to these components (23). Post-vaccination anaphylaxis is among the severe adverse effects of COVID-19 vaccines. However, it has been reported as an infrequent complication (2.5–4.8 cases/million doses in adults) (28). Interestingly, mRNA-based vaccines have caused more cases of post-vaccination anaphylaxis (4.8 cases/million and 5.1 cases/million after BNT162b2 and Moderna vaccines, respectively) (29), which could be attributed to the application of PEG2000 in these type of vaccines. Since post-vaccination anaphylaxis is a far less common adverse effect, the definition of a cause-effect relationship can be challenging and needs future studies to be focused on this occasion.
Capillary leak syndrome
Capillary leak syndrome (CLS) is the leakage of fluids in the vessels into the extravascular space. There are two types of classification for this disease: (1) idiopathic with unknown cause and secondary to underlying causes, (2) pulmonary CLS (PCLS), which mainly involves the lung and pleura, and systemic CLS (SCLS), which rarely consists of lungs edema (30, 31). Predisposing factors for secondary CLS include hematologic malignancies, medical treatment, and viral infections (32). In the last year, several case reports of developing pulmonary or systemic CLS following SARS-CoV-2 infection and vaccination (30, 33). Precise pathophysiology has yet needed to be elucidated. However, the assumed mechanism is dysregulation in inflammatory response leading to cytokine storm (i.e., viral sepsis) (31). High levels of pro-inflammatory agents and a hypoxic condition, pathogens' invasion, and immune cells' activity injure the epithelial-endothelial integrity causing permeability in the vascular wall and extravasation of exudative fluids (31). It has been demonstrated that hypoalbuminemia is a frequent finding in COVID-19 cases, representing the severity of injury to the epithelial-endothelial barrier (31). Both PCLS and SCLS have been reported after SARS-CoV-2 infection so far (30). However, CLS cases following COVID-19 vaccination had a previously diagnosed SCLS or suspicious history of SCLS symptoms (33, 34). SCLS generally leads to anasarca, hypotensive shock, hemoconcentration, hypoalbuminemia, monoclonal gammopathy, compartment syndrome, and multiple organ failure in more severe cases (33). It is worth noting that the diagnosis of SCLS is a diagnosis of exclusion (other causes of shock) (32). Lab data predominantly encompass hypoalbuminemia and increased level of creatinine, lactate dehydrogenase (LDH), creatine kinase (CK), and aspartate aminotransferase (AST) (18). The interval between vaccine administration and appearance of symptoms was about 1–2 days (18). For treatment, it has been suggested that the underlying conditions and ongoing damages should be managed. For example, albumin administration may even exacerbate the edema because vascular permeability is present continuously (18). Therefore, agents preserving epithelial-endothelial integrity can be beneficial, including solnatide, FX06, and Bβ15-42. Vasopressors (e.g., norepinephrine, vasopressin, and epinephrine), antibiotics, volume replacement, high dose of corticosteroid, IVIG (1 g/kg) are also pivotal. In the case of compartment syndrome, fasciotomy, and in the case of acute renal injury, renal replacement therapy (RRT) may be needed (18). oreover, prophylaxis with IVIG has been proposed as prevention in patients with prior SCLS who undergo vaccination (18).
Until today, several studies have reported SCLS as a complication of both COVID-19 and its vaccines (33–39). According to the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, SCLS has occurred in 13 cases among over 49 million shots of the ChAdOx1-S vaccine, and it has been advised not to use this vaccine in patients with a previous history of capillary leak syndrome (40). Since there is no clear evidence about the exact incidence of post-vaccination SCLS, at this moment, it is far too difficult to conclude that there is a cause-effect connection between SCLS and COVID-19 vaccination.
IgA vasculitis and leukocytoclastic vasculitis (hypersensitivity vasculitis)
Another worrisome issue observed following COVID-19 vaccination is the probability of new-onset emergence or exacerbation of pre-existing autoimmune diseases (41). There have been rare reports of IgA vasculitis reactivation, previously known as Henoch-Schönlein Purpura, following the COVID-19 vaccination (42–44). IgA vasculitis causes skin, joints, intestines, and kidneys' small blood vessels to inflate and bleed. The pathogenesis underlying IgA vasculitis is yet to be known completely. However, the role of genetic, environmental factors, vaccines, malignancies, and infections have been reported (45). The most common feature of the disease is the presence of purplish, especially on the buttocks and lower legs. Elevated CRP, ESR, Urea, Creatinine, serum amyloid A levels, IgM, IgA, and anti-spike IgG could suggest IgA vasculitis associated with COVID-19 vaccination. Methylprednisolone, Deflazacort, and Paracetamol were prescribed in the mentioned cases (42, 45). Similarly, cases of leukocytoclastic vasculitis were reported following COVID-19 mRNA-based vaccines. Histopathological evaluations and direct immunofluorescence analysis helped make the diagnosis of leukocytoclastic vasculitis. Prednisolone taper was prescribed for the mentioned cases (46–48).
In the case of 18F-FDG PET/CT imaging, only very few studies have utilized this method in the cases of vasculitis following COVID-19 infection (49, 50). In the study of Sollini et al. 18F-FDG PET/CT findings suggested that post-COVID-19 vasculitis could be considered a cause of prolonged symptoms (fatigue, dyspnea, and chest pain) following COVID-19 recovery (50). Nevertheless, the existing evidence is not enough to advise 18F-FDG PET/CT imaging for all cases, and future investigations must be conducted to determine whether it is rational to use this method in both post-COVID-19 and post-vaccination vasculitis.
Urticarial vasculitis
Urticarial vasculitis (UV) has been reported as a complication both after COVID-19 infection and the vaccination (51, 52). It is an inflammatory skin disorder manifested by the presence of urticarial rashes lasting more than a day and healing with hyperpigmentation. COVID-19 vaccine-induced UV is characterized by elevated red rashes on the skin, which are itchy. Elevated CRP and histopathological evaluations also help the diagnosis of the disease. In this case, oral indomethacin, levocetirizine tablet, and topical calamine lotion could be prescribed (52). Although some etiologies have been reported for UV, the idiopathic form is still the most common form of UV (53), and given that there are very few reports about both post-COVID-19 infection and post-COVID-19 vaccination UV, it is still far too soon to report a cause-effect relation.
Cutaneous vasculitis
Cutaneous vasculitis is an inflammatory disease that affects dermal blood vessel walls. The skin is usually involved. Cutaneous vasculitis can reflect a cutaneous component of systemic vasculitis, a skin-dominant or skin-limited expression or variant of systemic vasculitis, or be a single-organ vasculitis per se. The diagnosis is often based on physical examination and skin biopsy (54). The main histological finding is a recently reported peculiar post-COVID-19 vaccination maculopapular rash characterized by lymphocytic vasculitis. The rash responded to systemic antihistamine and local steroid therapy (55).
The exact incidence of post-COVID-19 vaccination cutaneous vasculitis has not been determined until now, and there are only a few case reports presenting this complication (56, 57). Interestingly, a recent case report by Uh et al. has presented five cases of cutaneous small-vessel vasculitis following the ChAdOx1-S COVID-19 vaccine (58). After all, like all the other rare adverse effects following COVID-19 vaccination, it faces us with a challenging question of whether there are any cause-effect relationships or not.
Rheumatoid arthritis and reactive arthritis
There have been reports of a rheumatoid arthritis flare-up following the COVID-19 vaccination. Moreover, rheumatoid arthritis exacerbation has also been reported after the COVID-19 vaccination (59), which had been previously observed following tetanus, rubella, hepatitis B, and influenza vaccines (60). The mechanism underlying this flare-up could be possibly attributed to the molecular mimicry or non-specific adjuvant effect. Elevated ESR and CRP levels with abnormal ultrasound evaluation of the swollen limb and arthrocentesis were suggestive of the flare-up of rheumatoid arthritis in these cases. Intra-articular steroids could be prescribed in these cases (61). Besides, a case of reactive arthritis has been reported following the SARS-CoV-2 vaccine in a 23-year-old woman treated with intra-articular betamethasone (59, 62). Despite the cases above, rheumatoid arthritis flare-up following the COVID-19 infection has also been reported (63).
IgA nephropathy
IgA nephropathy has been similarly reported following COVID-19 mRNA vaccines and the infection itself (64, 65). However, like most of the adverse events discussed in this paper, it is very uncommon, and establishing a cause-effect relationship is difficult and needs more investigation. IgA nephropathy is a complex immune disorder with IgA deposition in the mesangial layer. The “multi-hit hypothesis” is almost the most accepted underlying pathophysiology. An increased level of galactose deficient IgA1 (Gd-IgA1) is needed for the progression of IgA nephropathy and IgA vasculitis-induced nephritis. Then, IgG immunoglobulins, targeting these Gd-IgA1 antibodies, appear and form some immune complexes with the Gd-IgA1, leading to an inflammatory state. Nevertheless, the exact mechanism by which COVID-19 leads to the generation of Gd-IgA1 immunoglobulins is not entirely understood and needs more investigation (66). However, there are some possibilities. For example, since COVID-19 is a mucosal infection, it could lead to Gd-IgA1 formation through increasing IL-6 production, resulting in impaired IgA1 glycosylation/galactosylation (67).
Furthermore, in these cases, the development of IgA nephropathy could be speculated due to an elevated response of immune cells in the germinal center, leading to massive antibody production and increased production of pathogenic IgA similar to immunization with the influenza vaccine. The cases had gross hematuria. Urine analysis, Kidney ultrasound, evaluation of immunoglobulin A levels, and kidney biopsy were suggestive of IgA nephropathy in the mentioned cases. Losartan and methylprednisolone were prescribed in these cases (68–70).
Thyroiditis
Subacute thyroiditis, also known as De Quervain's thyroiditis, might be another rare adverse event with an immunological source associated with the COVID-19 vaccination (71–75). There are also a few reports about this complication after COVID-19 infection (76–78). It is a self-limited thyroid inflammation for weeks to months that commonly happens following a viral upper respiratory tract infection (79, 80). This possible post-vaccine effect has been presented with new-onset thyroid dysfunction in recently vaccinated individuals and appears to be of female predominance. The relationship between subacute thyroiditis and the type of COVID-19 vaccine is unclear as it has occurred following various vaccine platforms, including Sinovac, AstraZeneca, Bharat, Moderna, and Pfizer/BioNTech. This phenomenon was previously reported following H1N1, seasonal influenza, and hepatitis B vaccines (81–84). Vaccines' adjuvants are supposed to be responsible for these reactions by stimulating immunogenic cross-reactivity, causing autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome) (75, 85). After vaccination, subacute thyroiditis can develop due to ASIA syndrome, including COVID-19 vaccines (85). The development of ASIA syndrome could be attributed to molecular mimicry, polyclonal activation of B cells, and immunological imbalance of the host (75). In addition to ASIA syndrome, the interaction between SARS-CoV-2 spike protein with angiotensin-converting enzyme 2 (ACE2) receptor, which is wildly expressed on thyroid cells, is another mechanism associated with thyroiditis induction in vector-based vaccines such as AstraZeneca (72). Clinical manifestations associated with thyroiditis include pharyngitis, moderate fever, diffuse myalgia, and cervical pain that radiates to the jaw and ears. Subacute thyroiditis is often associated with negative anti-thyroglobulin and anti-thyroid peroxidase antibodies (80).
In the mentioned cases, suppressed thyroid-stimulating hormone (TSH) levels accompanied by elevated triiodothyronine (T3) and thyroxine (T4), increased levels of inflammatory markers (ESR, CRP), ultrasound findings, negative thyroid antibodies helped in diagnosing subacute thyroiditis. Methylprednisolone, propranolol, and ibuprofen were prescribed in these cases (75, 80, 86). In this relation, cases of Grave's disease have also been reported following receiving the SARS-CoV-2 vaccine with elevated anti-thyroid antibodies (87).
Dermal filler reaction
Hyaluronic acid (HA) is a natural polysaccharide that has been widely utilized in cosmetics (88). Reaction to HA fillers is rare and typically self-limited. So far, triggers such as infections (e.g., flu-like illnesses) and vaccinations (e.g., influenza vaccine) have been reported to exhibit filler reactions. Furthermore, several cases have been identified following anti-SARS-CoV-2 mRNA vaccines recently. Two cases with dermal fillers had developed swelling in lips and face in the third phase of the Moderna vaccine trial (89). There are two possible explanations for its pathophysiology. The first assumption is that the action of non-active components in vaccines cross-reacting with filler's molecules and provokes an immune response (90). The second hypothesis supports the alleviation of ACE2 conversion by mRNA vaccines, leading to pro-inflammatory ACE2 in the skin and inflammation. Reactions are type four hypersensitivity or delayed immune reactions mediated by T lymphocytes (90). Patients generally present with flu-like symptoms and swelling of the filler region days after vaccination. Tenderness, swelling, erythema, and nodules can be seen in the examination (90). Since most of the lesions have been resolved spontaneously, observation and follow-up are the first approach. However, in case of not improving nodules with pain, tenderness, or erythema, intervention is necessary (90). Antibiotics, including tetracycline and macrolides, should be administered for 3–5 days. If the nodule is non-inflammatory, hyaluronidase with or without intralesional steroids can be utilized to resolve it (90). Moreover, drainage of the nodule should be considered if the mass fluctuates. Interestingly, in some trials, low doses of ACE inhibitors have been used for 3–5 days, which significantly improved the reactions (90).
Systemic sclerosis
Systemic sclerosis (SSc) is a rare chronic inflammatory connective tissue disease that can cause vascular abnormalities, organ involvement, and skin fibrosis (91). Pulmonary fibrosis and pulmonary hypertension (PAH) are observed in most of these patients due to increased serum levels of TNF-α (92). Etanercept and infliximab are among the drugs that can reduce inflammation and improve the function of endothelial cells by reducing the serum level of TNF-α and preventing the progression of PAH and heart diseases (93). Due to the presence of lung fibrosis, the possibility of being infected with COVID-19 is high in these patients (94). In a case report, a 70-year-old patient with COVID-19 was investigated who developed diffuse cutaneous SSc 2 weeks after receiving the first dose of ChAdOx1 nCOV-19 vaccine. The patient had no history of contact with toxic substances. In the tests performed, ANA was positive and ENA was negative (95).
Vessel vasculitis
In the case report, a 71-year-old woman who was suffering from EGPA was treated with medicine for 7 years and has recovered to a great extent. The patient received the first dose of BNT162b2 mRNA vaccine 3 months after recovery (96). Ten days after receiving the vaccine, the patient was referred to the medical center complaining of increased myalgia and polyarthralgia, which appeared immediately after receiving the vaccine (96). The patient's clinical symptoms and conditions worsened continuously during hospitalization, but the RT-PCR is related to his COVID-19. It was always negative. CT scan of the patient had a magnifying glass view (96). Also, laboratory tests such as CRP, ESR, p-ANCA and Birmingham Vasculitis Activity Scores (BVAS) were above the normal range. The patient was treated with corticosteroids and was discharged after 12 days of hospitalization. First, she was treated with intravenous pulse methylprednisolone for 3 days and then was treated with oral prednisone for 6 months (96).
In another report, it is stated that a 46-year-old man went to the hospital a week after receiving the second dose of Pfizer BioNTech COVID-19 vaccine, complaining of severe abdominal pain since 6 days ago (97). The first day after receiving the vaccine, the patient had fever and seizures. The patient's CRP level was slightly higher than normal (97). Serological panels for autoimmune and vasculitis were negative But in the CT scan, inflammation indicating focal vasculitis was observed, and MRA confirmed these observations. The patient was treated and discharged from the hospital after 1 week, but was still under follow-up for 9 months. The patient was treated with steroids in the first 6 months of follow-up and azathioprine 150 mg daily in the next 3 months and completely recovered (97).
Renal complications
Kidneys can be widely affected by SARS-CoV-2 due to the high expression of ACE2, which is expressed more in proximal convoluted tubules (PCTs). Therefore, the most common type of injury following COVID-19 infection is tubular injury. Other reported clinical pictures include nephrotic syndrome and glomerular injury (e.g., minimal change disease) (98, 99). Several case reports of acute kidney injuries in patients with or without prior renal pathology following vaccination (100–102). However, it has been reported that vaccination could exert a protective effect against acute kidney injury. While the infection was associated with an increased risk of acute kidney injury (125.4 events per 100,000 persons), vaccination with BNT162b2 mRNA vaccine could reduce the risk (−4.6 events per 100,000 persons) (103). The pathophysiology of SARS-CoV-2-related renal injury is presumed to be multifactorial through direct (i.e., virus or vaccine components) or indirect (i.e., immune-mediated like cytokine storm or hyperactivity of T cells) effects (104). After vaccination, suspension to kidney injury encompasses manifestations like oliguria or anuria, edema or anasarca, hypertension, and dyspnea due to pleural effusion (98). Laboratory data confirming the diagnosis include a variety of renal-associated factors' impairment due to underlying pathology. For instance, minimal change disease exhibits proteinuria, normal to increased creatinine, and podocyte injury in a light microscopy assay (101). Acute tubular necrosis can be presented with increased creatinine and urea nitrogen, proteinuria, hypoalbuminemia, biopsy findings of diffuse PCT injury, lymphocyte infiltration, and cell necrosis (98, 99).
Furthermore, hypodensity of renal parenchyma may have been seen in computed tomography (CT) (104). Treatment consists of two important approaches; firstly, kidney protection from further injury by adjusting input and output fluids, excluding nephrotoxic drugs, and monitoring creatinine levels. Secondly, early immunosuppressive therapy (non-selective or selective) or RRT due to the patient condition (104, 105).
Dermatologic complications
Erythema multiforme
Erythema multiforme (EM), an inflammatory dermatologic disorder, is linked chiefly to infections (most commonly: herpes simplex and Mycoplasma pneumoniae), although various triggers, such as many other infectious agents, immunizations, medications, and even various diseases, have also been identified. Acral, targetoid papules, consisting of three distinct concentric zones, are the hallmark lesions of this disease. It is necessary to emphasize that vaccine-induced EM has been known for a long time, with 984 cases reported to the Vaccine Adverse Event Reporting System (VAERS) (106). Furthermore, both as typical acral lesions in younger individuals and more widespread, atypical lesions in adults, EM-like reactions have already been linked to SARS-CoV-2 infection (107). The structure codified in mRNA COVID-19 vaccines, SARS-CoV-2 spike protein, was shown immunohistochemically in the epithelium and endothelial cells in the eccrine ducts in those individuals. EM is a rare adverse effect of many other vaccines, and recent studies link this reaction to mRNA vaccines (108). It has been suggested that a T-cell trigger by viral antigen-positive cells containing the HSV-DNA polymerase gene plays a vital role in EM pathogenesis, and it causes viral gene expression in the recruitment and skin (109). EM's clinical manifestations are diverse, and they can also manifest as atypical palpable lesions with erythematous dusky bodies surrounded by a paler halo. To rule out inflammatory, autoimmune, or malignant disorders, swabs are performed for HSV-PCR, Tzanck smear, or other serological tests. Direct and indirect immunofluorescence may help differentiate EM and distinguish it from other lesions of bullous vesicles. EM is managed with symptomatic treatments. The lesions may heal in 3 to 6 weeks, but patients with severe EM may need to be hospitalized for antiviral therapy, hydration, analgesics, and systemic steroids (106, 109).
Chilblains
Repeated exposure to cold air may cause inflammation in small blood vessels, called chilblains, causing swelling, blistering, itching, and red patches on hands and feet (110). The exact pathophysiology remains unclear since the chilblain-like lesions due to COVID-19 are common features with idiopathic and autoimmune-related chilblains. COVID-19 has various clinical manifestations, including pernio/chilblains-like lesions, a condition termed “COVID-19 toes.” However, after receiving the vaccines, this condition might be associated with COVID-19 (111). These lesions have been reported post-Pfizer and -CoronaVac (inactivated vaccine) vaccinations (112, 113). These lesions could be extremely painful and last for up to 150 days after vaccination (114, 115). Anticoagulant therapy (apixaban) and low-dose aspirin were prescribed for the patient until circulating immune complexes were obtained after 14 days (114).
Hematologic complications
Disseminated intravascular coagulation
Disseminated intravascular coagulation) DIC) leads to extensive fibrin deposition with the formation of extensive microvascular thrombosis (116). During coagulation, coagulation factors decrease, and platelets accumulate, thereby reducing clotting protein (116). Infection by the SARS-CoV-2 increases the risk for systematic multi-organ complications and venous and arterial thromboembolism (116). CT scan demonstrated multiple subacute intra-axial hemorrhages in atypical locations, such as the right frontal and temporal lobes. A successive CT angiography of the chest added the findings of multiple contrast-filling defects with multi-vessel involvement: at the level of the left interlobar artery, of the right middle lobe segmental branches of the left upper lobe segmental branches, and the right interlobar artery (116). A plain old balloon angioplasty (POBA) of the right coronary artery was conducted, with the restoration of distal flow but with the persistence of extensive thrombosis of the vessel (116). An abdomen CT angiography demonstrated filling defects at the right supra-hepatic vein level and the left portal branch level. Bilaterally, it was adrenal hemorrhage and blood in the pelvis (116). An MRI on the same day demonstrated the presence of an acute basilar thrombosis associated with the superior sagittal sinus thrombosis. Alternative HIT-compatible anticoagulants prescribe in case of acute thrombocytopenia/thrombosis (116).
Deep vein thrombosis and pulmonary thromboembolism
Deep venous thrombosis (DVT) and pulmonary thromboembolism (PTE) exist on the spectrum of venous thromboembolic disease (VTE) (117). DVT is known as the formation of blood clots (thrombi) in the deep veins. It usually affects the deep leg veins (e.g., the calf veins, popliteal vein, or femoral vein) or the deep veins of the pelvis. DVT is a potentially dangerous condition, leading to preventable morbidity and mortality (118). PE occurs when a thrombus migrates from the venous circulation to the pulmonary vasculature, lodging in the pulmonary arterial system. The clinical manifestation of acute PE ranges from incidentally discovered and asymptomatic to massive PE, leading to death (117). Post-vaccination DVT and PTE have been reported in a few cases worldwide. Infection could significantly increase the risk of DVT (RR of 3.78; 43.0 events per 100,000 persons) and PE (RR of 12.14; 61.7 events per 100,000 persons). Nonetheless, the BNT162b2 mRNA vaccine was not associated with an increased risk for these adverse effects (RR of 0.87; −1.1 events per 100,000 persons and RR of 0.56; −1.5 events per 100,000 persons for DVT and PE, respectively) (103). Duplex ultrasonography of the lower limbs demonstrated acute DVT involving the superficial femoral, common femoral, popliteal, anterior tibial, posterior tibial, and deep calf veins (119). The patient underwent computed pulmonary tomography angiography (CTPA) due to tachycardia, which demonstrated saddle thrombus in the bifurcation of the pulmonary trunk and 40 extensive bilateral main pulmonary arteries emboli extending to both lobar segmental and sub-segmental branches (119).
Sporadic cases report viral vector vaccine injection, vaccine-induced immune thrombotic thrombocytopenia (VITT), and cerebral venous sinus thrombosis (CVST). Generally, CVST occurs in young adults, particularly young women. In most cases, a risk factor is identified in patients (120–123). With disease progression, focal neurological deficits may develop due to seizure and venous infarction, more commonly observed in patients with CVST than the other stroke subtypes. Full recovery is achievable with timely disease diagnosis and treatment (124). The SARS-CoV-2 infection has also been proved to lead to CVST development in several studies (124, 125). SARS-CoV-2 VITT is a novel phenomenon that might occur in post-viral vector COVID-19 vaccines.
Contrary to the previous reports of post-vaccination thrombotic thrombocytopenia, CVST is reported in these patients after the COVID-19 vaccination. Clinically, VITT mimics spontaneous autoimm